
8
Experiment 3. Influence of dilution on buffer capacity
In a flask, prepare 15 ml of acetate buffer with pH
0
= 5 (mix 9.6 ml 0.1 mol/l
CH
3
COONa and 5.4 ml 0.1 mol/l CH
3
COOH). 5 ml of the solution is transferred
to another flask and add to this volume 5 ml of distilled water. Both solutions
are gently titrated with 0.1 M NaOH in the presence of a methyl red indicator
(3 drops) until the lemon-yellow color appears (pH = 6.2). Titration is repeated
2 times. The average alkali volume is taken for calculation. Calculate the buffer
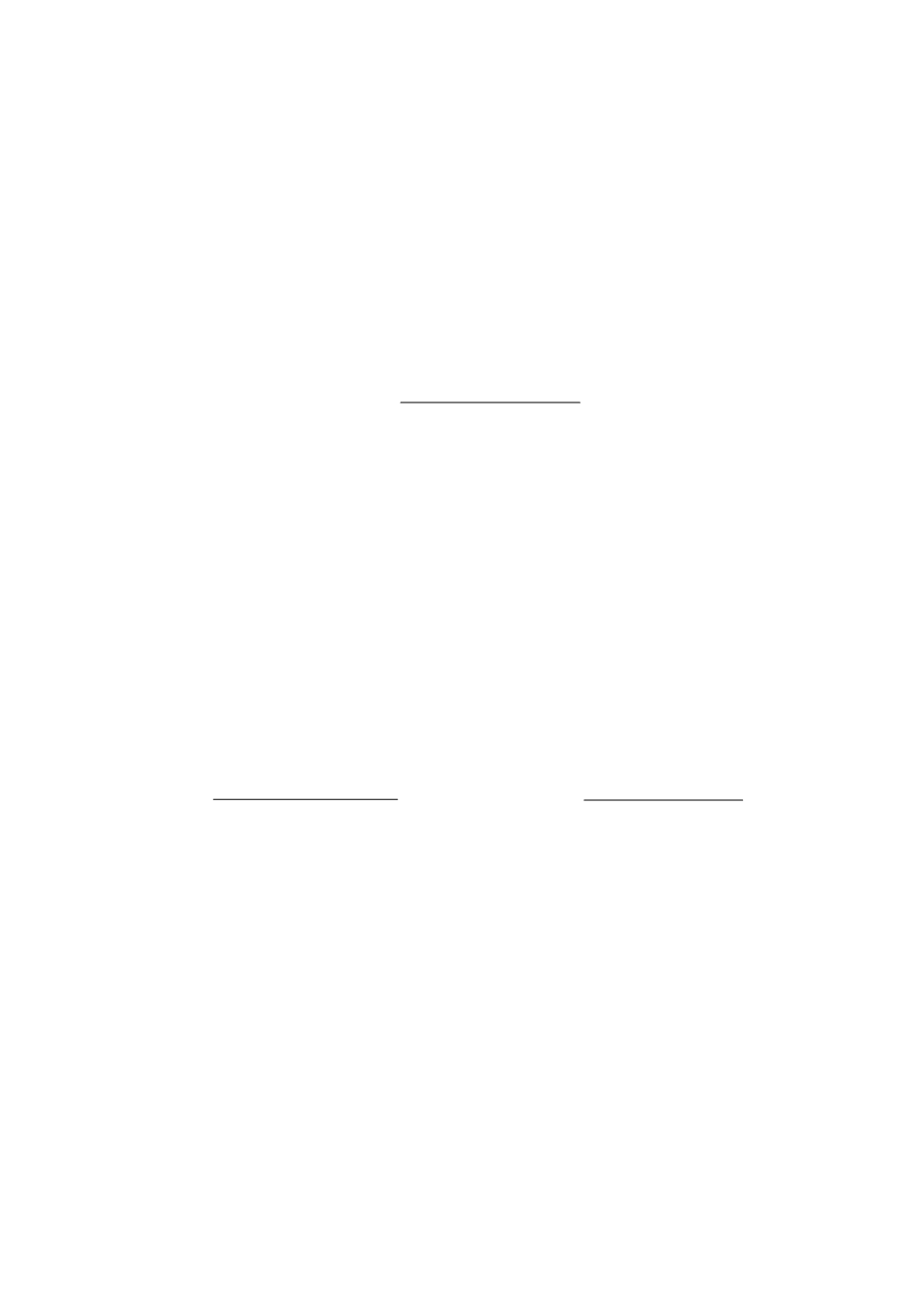
capacity of solutions in alkali (mmol/l) according to the formula:
С
NaOH
– normality of alkali solution, mol/l; V
NaOH
– volume of alkali solution needed for
titration, l; V
buff
is the volume of the buffer solution, l; pH
0
and pH
1
– the pH value of the
buffer, respectively, before and after the addition of alkali to it.
Experience № 4. Determination of the buffer capacity of blood serum
Pour 5 ml of blood serum (рH
0
= 7.36) in two flasks for titration. Then two
drops of phenolphthalein indicator are added to one flask and titrated with 0.1 M
NaOH until a weak pink staining (pH
1
= 9.1). Two drops of methyl orange are
added to the other flask and titrated with 0.1 M HCl until orange-pink staining
appears (pH
2
= 3.7). Calculate the buffer capacity of blood serum for alkali and
acid by the formulas:
Based on the results obtained, make a conclusion.
LESSON 4.
COLLATIVE PROPERTIES OF SOLUTIONS:
LAWS OF RAUL AND VANT-GOFF
Required base level.
Molarity, molality, mole fraction. The boiling point and freezing point of
water. Dissociation of acids, bases, salts.
Questions for the preparation for the lesson.
1. Colligative properties of dilute solutions of nonelectrolytes: Raoult’s
laws, Van’t Hoff’s law.
NaOH NaOH
1
0
1000
(рН рН )
alk
buff
С V
b
V
⋅
⋅
=
−
NaOH NaOH
1
0
1000
(рН рН )
alk
buff
С V
b
V
⋅
⋅
=
−
HCl
HCl
0
2
1000 ;
(рН рН )
acid
б
С V
b
V
⋅
⋅
=
−









