
10
and determine its freezing point (
t
1
). The freezing point of the water is known
(
t
0
). Calculate ∆
t
=
t
0
‑
t
1.
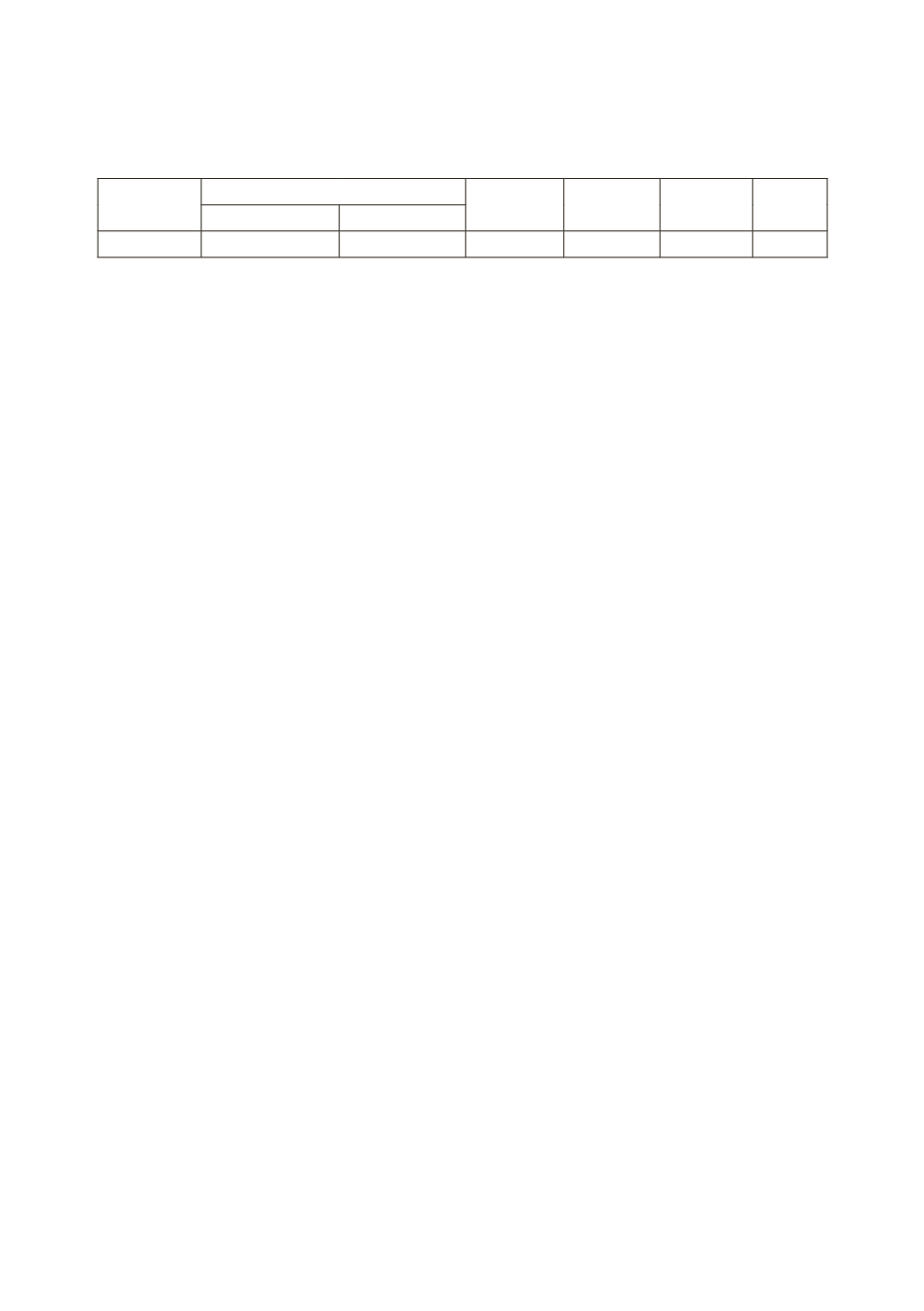
The obtained data is summarized in the table:
Substance
Concentration of solution
t
0
t
1
∆
t
M
mass fraction molality
Writea detailed calculation of the molar mass of the substancein the report.
LESSON 5.
HETEROGENEOUS EQUILIBRIUM AND PROCESSES
Required base level.
Soluble and insolublesubstances, solubility. Formation of precipitation in
chemical reactions.
Questions for the preparation for the lesson.
1. Heterogeneous reactions in solutions of electrolytes. Solubility constant.
Conditions for the precipitateformation and its dissolution.
2. Reactions of formation of the basic inorganic substance of bone tissue of
calcium hydroxide phosphate.
3. The mechanism of the functioning of the calcium-phosphate enamel
buffer of the teeth.
4. The isomorphism: replacement of hydroxide ions by fluorine ions,
replacement of calcium ions by strontium ions in hydroxylapatite.
Input test-control on the topic «Heterogeneous equilibria».
Homework exercises.
1. Write the expressions for the solubility constants KS of the following sub-
stances: SrSO4, Ag2CrO4, BaCO3.
2. Write the equations of reactions underlying the formation of hydroxylapa-
tite, the basic inorganic substance of bone tissue.
3. Write the equation of the reaction underlying the buffering action of the
enamel of the teeth.
4. The solubility of Mg (OH)2 at 18 °C is 1.7×10–4 mol/l. Calculate the
solubility constant Mg (OH)2 at this temperature.
5. It is known that KS (Ag2SO4) = 7.7×10–5. Determine the solubility of
silver sulphate in mol/l and in g/l.
6. Is a PbCl
2
precipitate formed by mixing a 0.05M Pb (NO
3
)
2
solution with an
equal volume: a) a 0.05M HCl solution; b) 0.5 M solution of HCl?
K
S
(PbCl
2
) =
1.7×10
–5.
Prove this by calculation.









