
7
will the pH value change when the solution is diluted two-fold? Is it possible to
prepare acetate buffer with pH = 6.5 if K
a
(CH
3
COOH) = 1.75×10
–5
?
4. What volumes of solutions of ammonium hydroxide (C = 0.05 mol/l)
and ammonium chloride (C = 0.05 mol/l) should be taken to prepare 1 liter of
0.05 M ammonia buffer pH 8.54? pK
b
= 4.76.
5. The solution of HCl (1.5 ml of a 0.02 mol/l) was added to 1.5 ml of blood.
The pH value was changed from 7.4 to 7.2. What is the buffer capacity of blood
for acid?
Questions for self-study.
Gas solubility: Henry’s law, Dalton’s law, Sechenov’s law.
Laboratory work:
Experience number 1. Preparation of acetate buffer systems
In three tubes, prepare 5 ml of acetate buffer solution, mixing the solutions
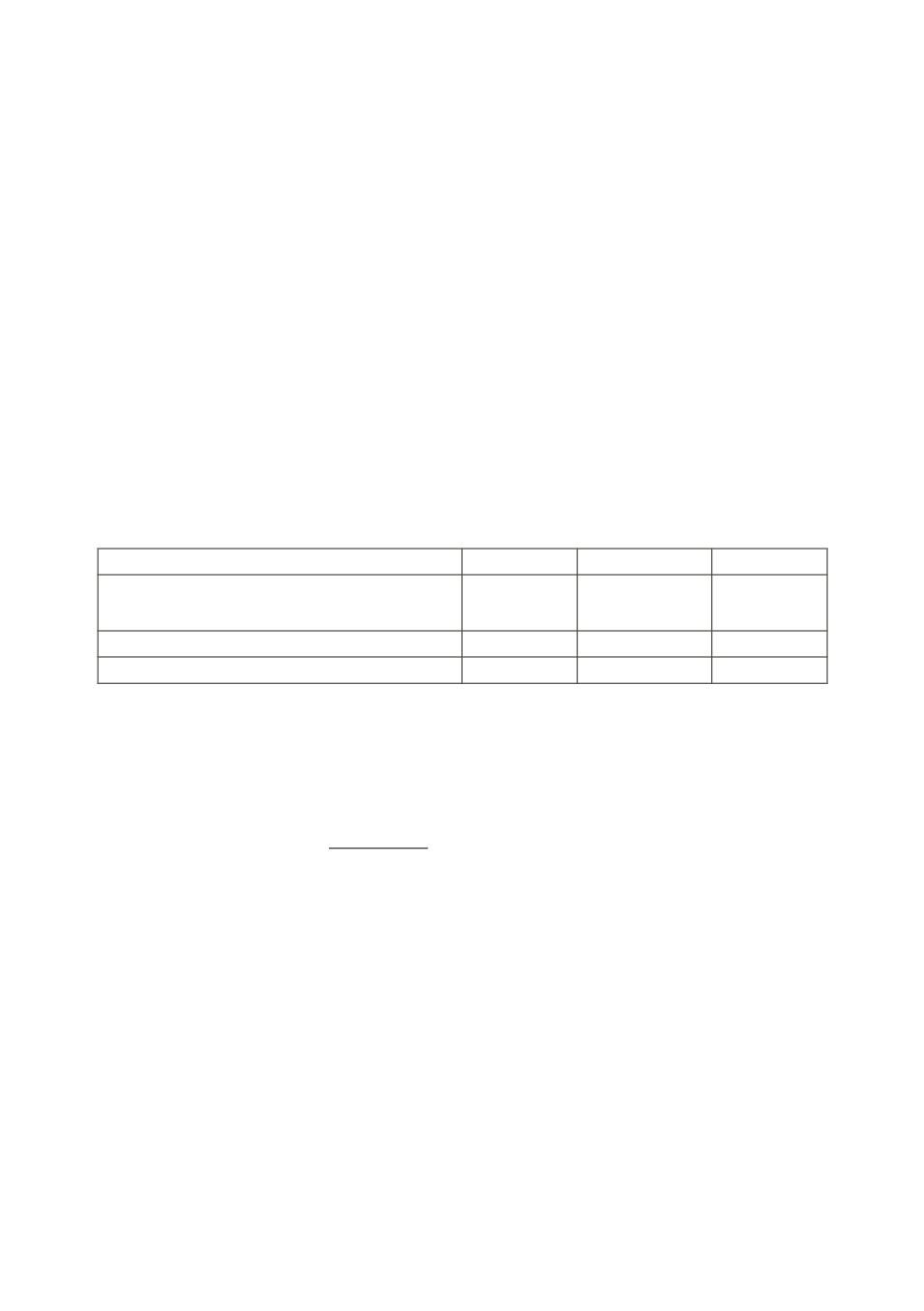
of 0.1 M acetic acid and 0.1 M sodium acetate according to the table:
No. of mixture
1
2
3
V
(0.1 mol/l СН
3
СООН), ml
V
(0.1 mol/l СН
3
СООNa), ml
0,5
4,5
1,5
3,5
4,5
0,5
Methyl red
pH
Solutions of acid and salt are taken from burettes. Add 3 drops of the indica-
tor – methyl red in each tube and observethe color in the table.
Calculate the pH of each of the three buffer solutions using the Henderson-
Hasselbach equation:
рН р lg
solt
solt
а
acid acid
С V
К
С V
⋅
= +
⋅
, where pK
a
(CH
3
COOH) = 4.76.
The results of calculations are entered in the table.
Experiment 2. The effect of acid, alkali and dilution on the pH of the
buffer solution
In four tubes, prepare 2 ml of acetate buffer solution: mix 1 ml of 0.1 M acetic
acid and 1 ml of 0.1 M sodium acetate (pour from the respective burettes).).
Then add 5 drops of 0.1 M HCl to the first of the tubes, add 5 drops of 0.1 M
NaOH tothe second tube, and add 5 drops of distilled water to thethird tube, and
leave the fourth tube for comparison.
In each tube add 2 drops of the indicator – methyl red. Compare the color of
solutions in four test tubes. Make a conclusion.









